Carbon fixation is the vital function plants, algae and some bacteria perform in removing carbon dioxide from the atmosphere, by adding energy and converting it to sugars needed for life function. Weizmann Institute of Science researchers in the laboratory of Ron Milo wanted to investigate how this process might be harnessed and reprogrammed into organisms higher in the food chain than those that normally perform this function.
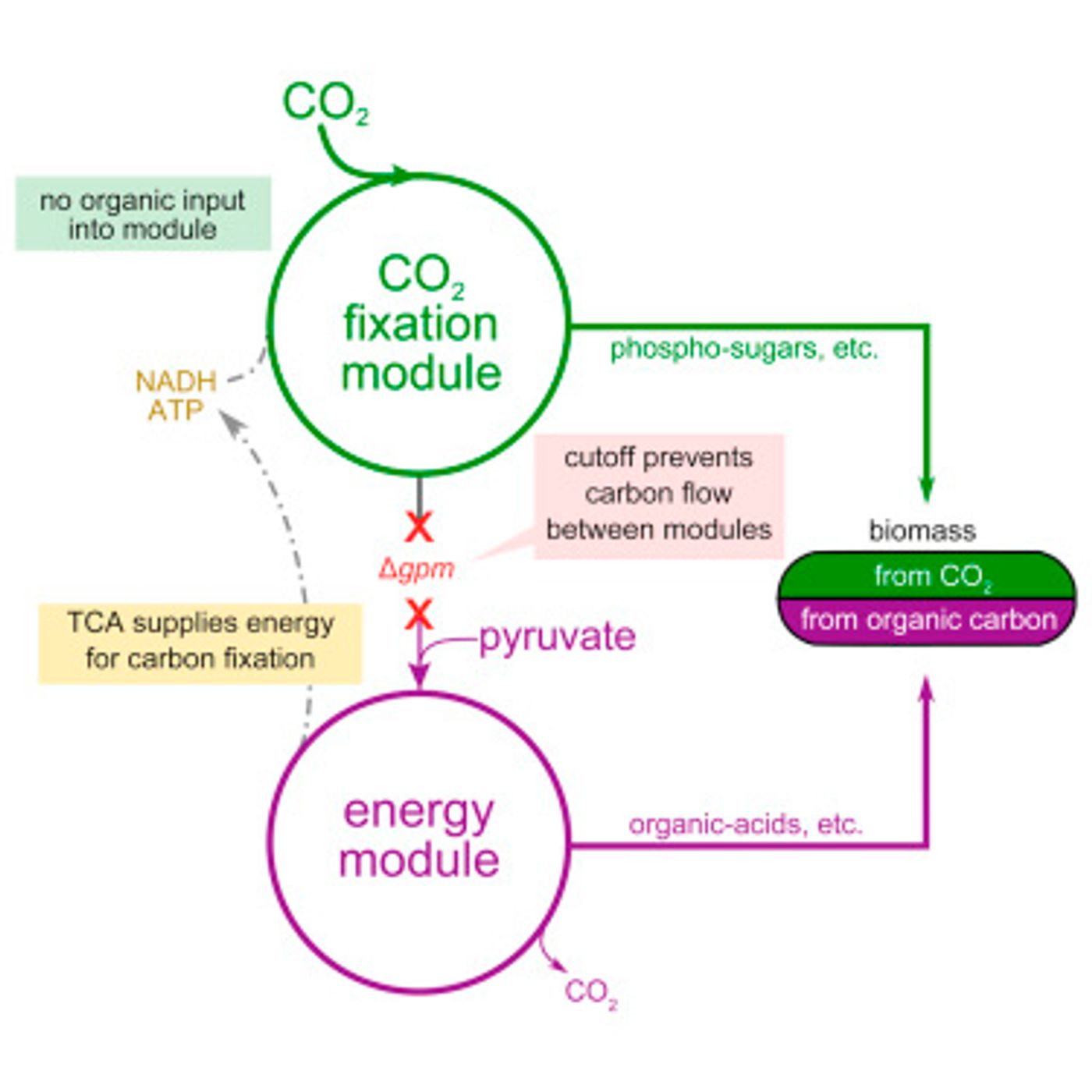
The researchers think that improving carbon fixation could be key to coping with challenges the planet will face in the future, for example the need to provide enough food for the expanding global population on shrinking arable land resources, all while consuming less fossil fuel. A schematic of their idea is shown above in the graphical abstract of the
paper, from Cell.
To tackle this problem, the scientists decided to take the Calvin cycle - the metabolic pathway for carbon fixation and sugar production – and insert it into E. coli, a bacteria known to consume sugar and release carbon dioxide. The Calvin cycle in described in the video below.
Since the metabolic pathway for carbon fixation is so well characterized, Milo and his team expected that the genes that contain the information for building the Calvin cycle could simply be spliced into the genome of the bacterium. However, a problem arose. A critical enzyme used to fix carbon in plants needs a substrate to function, a substrate which just happens to be toxic to the bacteria. The design then had to also include precise regulation of the various genes in this multistep pathway.
The bacteria weren’t very cooperative though. While they did produce functional enzymes needed for the Calvin cycle, they didn’t want to eat the carbon dioxide. They instead turned to a different sugar supply. "Of course, we were dealing with an organism that has evolved over millions of years to eat sugar, not CO2," said first author of the study, Niv Antonovsky. "So we turned to evolution to help us create the system we intended."
The team created tanks called "chemostats" for growing the bacteria. At first the bacteria in the tanks were offered carbon dioxide along with a large amount of pyruvate, an energy source, supplemented with just enough sugar to survive. Thus the microbes learned, through environmental pressures and stress, to develop an appetite for carbon dioxide. It did take three months for the bacteria to begin to thrive on their new energy source, but they were finally ready to be weaned from sugar completely and live on carbon dioxide and pyruvate alone. Through isotope labeling, it was confirmed that the bacteria were in fact using carbon dioxide to make both a significant portion of their body mass, and all the sugars required to make the cell.
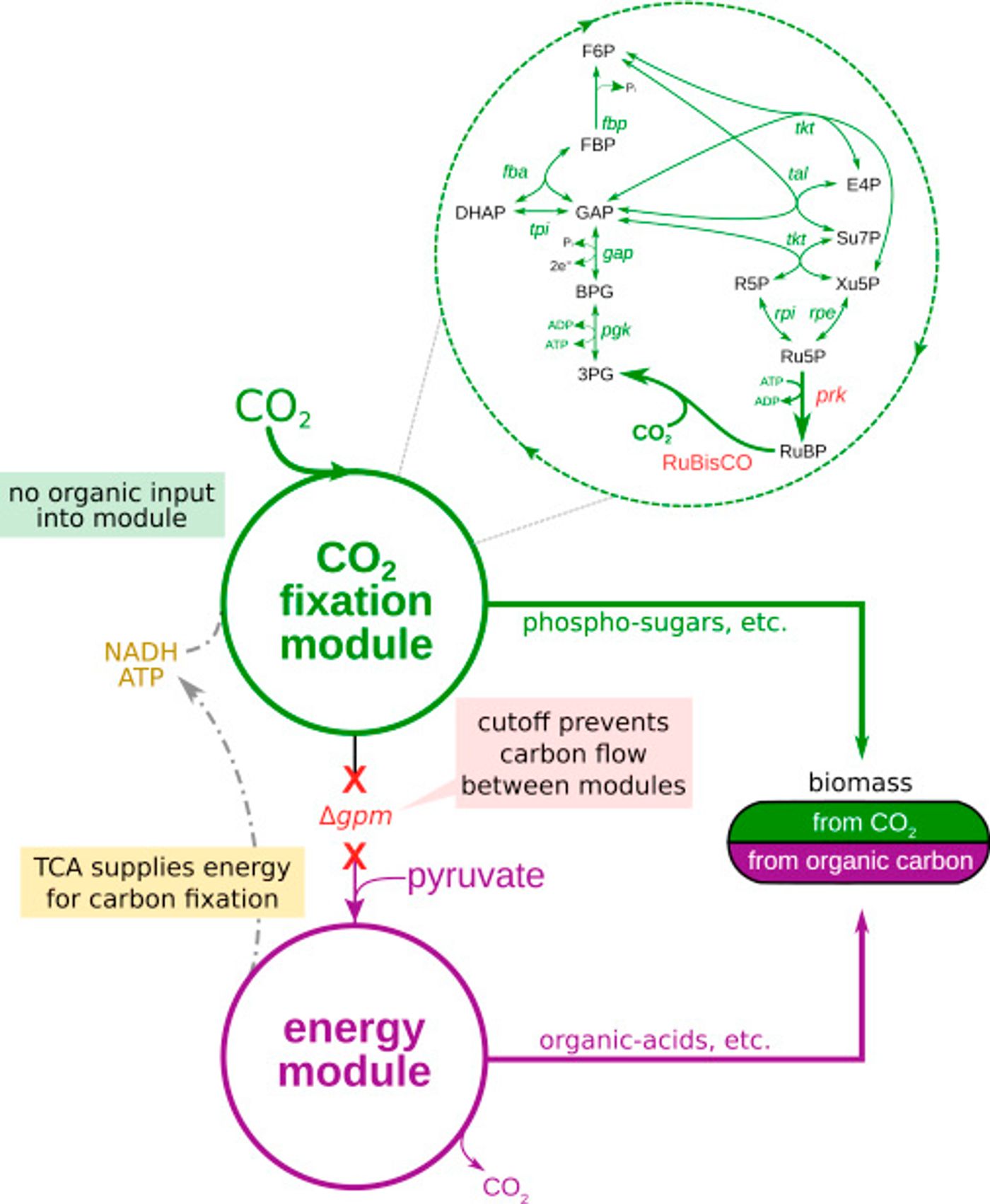
Image from Cell
After the genomes of the evolved bacteria were sequenced, the investigators detected many changes throughout the chromosomes. "They were completely different from what we had predicted," commented Milo. "It took us two years of hard work to understand which of these are essential and to unravel the 'logic' involved in their evolution." Repeating the experiment took months but provided essential information about what mutations are necessary for changing the E. coli diet from being sugar based to carbon dioxide based.
Milo remarked, "The ability to program or reengineer E. coli to fix carbon could give researchers a new toolbox for studying and improving this basic process." While the bacteria currently release carbon dioxide back into the atmosphere, the researchers hope that in the future their work could be applied to creating microbes capable of absorbing atmospheric carbon dioxide or to engineering crops that contain carbon fixing pathways, which may result in increased yields and better adaption to feeding the world.
Sources:
Science Daily via
Weizmann Institute of Science,
Cell