Operating Lithium Battery Mechanism Details Revealed
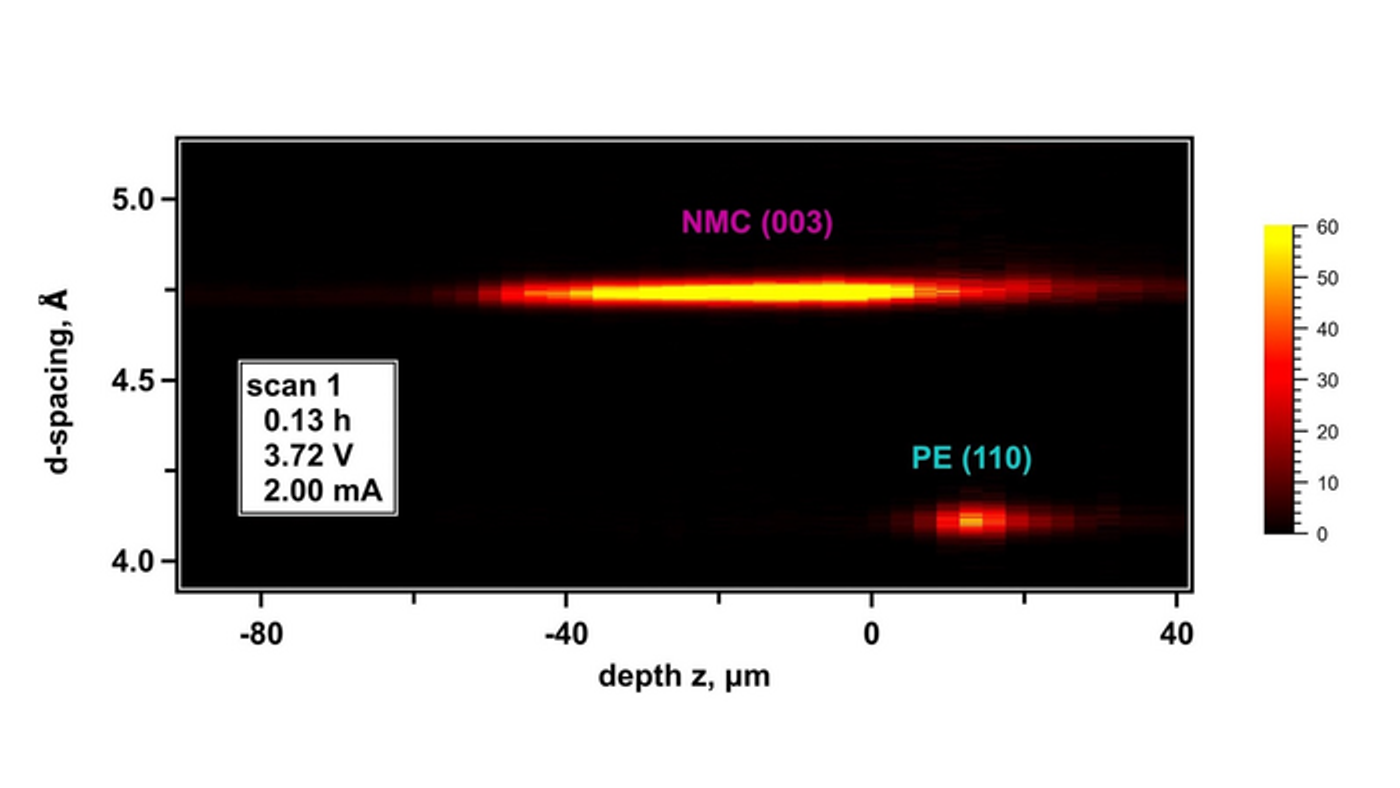
What goes on inside batteries? This what a team of researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory hope to find out as they used a novel X-ray method to observe the component movements within an operating lithium battery. This includes when the battery both charges and discharges approximately down to a millionth of a meter, and study holds the potential to help increase the energy density of battery cells, as countless batteries are being produced for both energy storage and electric vehicles that could help mitigate the impacts of climate change.
Still image from a animation demonstrating an operating battery cell's movements. (Credit: Argonne National Laboratory)
“It’s really exciting to be able to visualize these movements,” said Dr. Daniel Abraham, who is a senior materials scientist in Argonne’s Chemical Sciences and Engineering (CSE) division, and a co-author on the study. “Other researchers have previously guessed that these movements happen, but they’ve not been able to show them in such fine detail.”
At present, lithium-metal batteries hold the most promise as their negative electrode is comprised of a lithium metal anode as opposed to a traditional graphite anode. While these lithium metal anodes possess an energy storage capacity of an order of magnitude greater than traditional graphite anodes, the many charge-recharge cycles result in reduced performance in the long-term.
The charging of a lithium metal battery cell consists of a back-and-forth process where lithium ions transfer from a cathode to an anode by means of an electrolyte. During this process, the anode experiences expansion and contraction as the lithium atoms are deposited and then removed from the anode’s surface.
“An ideal lithium battery would reversibly deposit and strip a uniform layer of lithium over thousands of charge-discharge cycles,” said Abraham. “Such a battery would store and deliver charge for many years.”
While an interphase layer can be used to shield the health of the battery with the job of separating the lithium metal and electrolyte to prevent a reaction between them. However, the battery cell would cease to function since this process causes the electrolyte to be consumed. Also, too thick of an interphase layer results in small pores forming as the layers stack on top of each other when the anode receives new deposits of lithium during the process.
“If you’re trying to design batteries for phones, computers and cars, you don’t want the irreversible expansion of the anodes to be too much,” said John Okasinski, an Argonne physicist in the X-ray Science division and one of the study’s authors. “That could cause problems with other components in the device.”
The study’s findings indicate that the advanced X-ray method used here can be used to quantify the reversible lithium that is both deposited and removed, along with measuring the amount of lithium metal the interphase layer permanently takes. These findings can be therefore used to screen for the best electrolytes.
“Battery scientists can use the technique to quickly identify the best electrolytes,” said Abraham. “These would be the ones that form a thin interphase layer, prevent the mossy pileup of material and allow lithium metal to deposit and dissolve uniformly. One of our next research steps is to use the technique to evaluate how next-generation electrolytes can optimize lithium deposition and the interphase layer. We also plan to investigate how different anode surfaces — like copper — can alter and improve these processes.”
What new discoveries will scientists make about lithium batteries in the coming years and decades? Only time will tell, and this is why we science!
Sources: Journal of Power Sources, Argonne National Laboratory
As always, keep doing science & keep looking upI