A Genetic Edit Shields Cells That Are Usually Destroyed by Type 1 Diabetes
Diabetes is a disorder in which the body cannot properly regulate blood sugar levels because of a problem with insulin, a hormone that enables the body to get sugar out of the blood and move it into cells. In type 2 diabetes, the body either doesn't make enough insulin or doesn't respond to it properly, and is thought to be due to a combination of genetic and environmental factors. Type 1 diabetes, however, is a disease in which the immune system attacks the cells in the pancreas that produce insulin. Those with the disorder completely lack insulin, and they have to carefully manage their blood sugar for their entire lives. It can lead to other health problems including heart disease and may shorten life expectancy by over a decade.
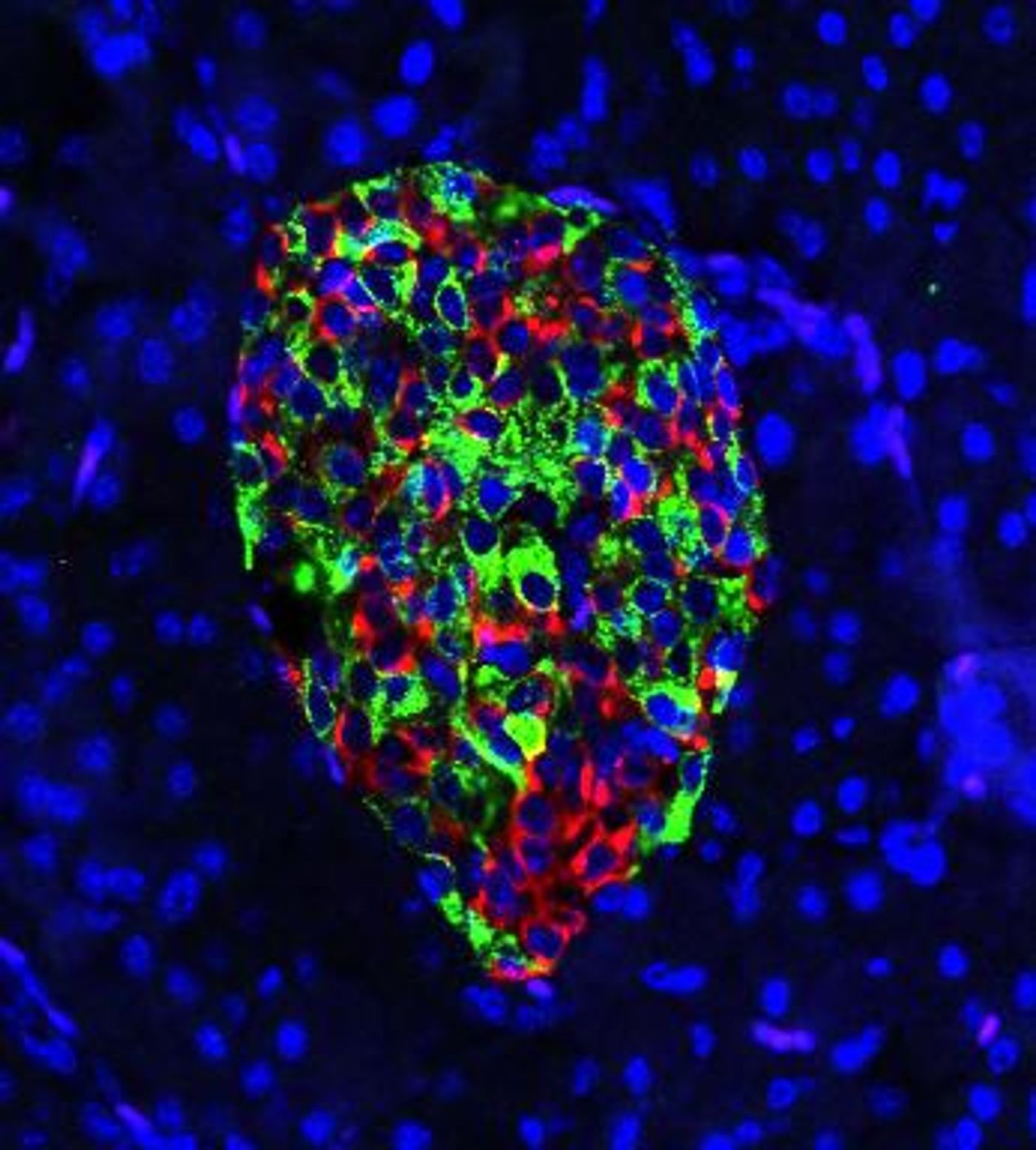
Researchers have now found that by removing a gene called IRE1-alpha from cells that produce insulin, called beta cells, in a mouse model of type 1 diabetes, the immune system stops attacking these cells. That relieves the symptoms of the disease because the cells can start producing insulin again. The findings, which have been reported in Cell Metabolism, suggest that it may be also possible to prevent other diseases in which the immune system mistakenly attacks the body's cells.
"The thing is, individuals who are at high risk can be identified," noted the lead author of the report, Feyza Engin, a biomolecular chemistry professor at the University of Wisconsin-Madison. "They have autoantibodies in their blood serum, meaning we can actually tell who is going to develop type 1 diabetes within a couple of years. But there's not much for clinicians to do but send them home, because there's no cure for type 1 diabetes."
The IRE1-alpha gene plays a role in how the cell responds to stress. The researchers expected that deleting this gene from beta cells in their type 1 diabetes mouse model would accelerate the progression of the disease. They were surprised to find something else happening.
"We expected the beta cells would die soon," Engin said. "Instead, my students told me that the blood glucose levels of the mice were becoming normal following an initial increase lasting a couple of weeks. I couldn't believe it. I said, 'What? No. Just measure it one more time.'"
Instead of being destroyed by the immune system's T cells, the beta cells were making insulin as they should normally. They were also becoming a bit less mature. If this so-called de-differentiation occurs before the T cells launch an attack, the interaction between the cells changes.
"Once we remove this gene, it's almost like the beta cells are undergoing a disguise," explained Engin. "They lose their mature identity. They de-differentiate and exhibit features of progenitor cells, and express hormones of other cell types in addition to insulin. When they de-differentiate, they don't act like typical beta cells anymore. They reduce the expression of many genes that signal to immune cells, 'Come and eat me!' Those signals go down, and that actually is altering the diabetogenic activity of T cells. They don't really recognize the beta cells as a problem anymore. They don't attack."
Importantly, the researchers found that the immature beta cells begin to mature again into totally functional beta cells.
"The mice experienced a little transient hyperglycemia. They have relatively high blood sugar, which isn't dangerous, for a few weeks," added Engin. "But then the beta cells get back to work, and make insulin like they're supposed to."
The scientists have continued to follow their mouse model, and have found that the T cells don't attack the beta cells again.
"That's the beauty of it," Engin said. "Even after the beta cells come back, the T cells leave them alone. They still have no diabetogenic activity one year later, which is like 40 or 50 years in a human life."
This research can now help scientists develop drugs that exploit this mechanism.
Sources: AAAS/Eurekalert! via University of Wisconsin-Madison, Cell Metabolism