Microbiology
UCI Researchers Study Way to Block Botulism
JUL 17, 2014 12:00 AM PDT
Share
Aging Population Has Urgent Need for Alzheimer
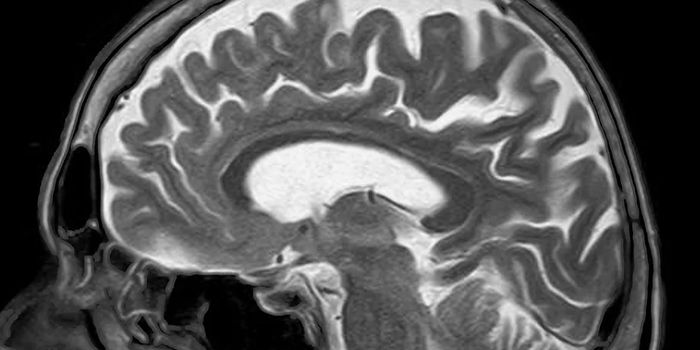
 As the population ages, victims of Alzheimer's disease (AD) are growing in number at a very fast rate. It's estimated that 44 million people worldwide are afflicted with the disease now and that this number will reach 100 million by 2050. Yet, there are only five drugs approved for treatment of AD. Four of them are cholinesterase inhibitors and one is an N-methyl-D-aspartate (NMDA)-receptor antagonist. Cholinesterase inhibitors work by preventing the Alzheimer's-caused breakdown of acetylcholine in the brain, a chemical messenger that helps memory, alertness and judgment. Memantine, the NMDA-receptor works by regulating the activity of glutamate, another chemical messenger involved in brain functions such as learning and memory.
As the population ages, victims of Alzheimer's disease (AD) are growing in number at a very fast rate. It's estimated that 44 million people worldwide are afflicted with the disease now and that this number will reach 100 million by 2050. Yet, there are only five drugs approved for treatment of AD. Four of them are cholinesterase inhibitors and one is an N-methyl-D-aspartate (NMDA)-receptor antagonist. Cholinesterase inhibitors work by preventing the Alzheimer's-caused breakdown of acetylcholine in the brain, a chemical messenger that helps memory, alertness and judgment. Memantine, the NMDA-receptor works by regulating the activity of glutamate, another chemical messenger involved in brain functions such as learning and memory. Although these drugs can help manage symptoms, such as memory loss and reasoning problems, none of them comes close to a cure or stops the disease's progression. Other problems with the drugs are that they do not work for everyone and their effects wear off over time. None of the drugs are approved for mild cognitive impairment (MCI), which is often, but not always a precursor to Alzheimer's, because clinical trials have not shown that they are beneficial for preventing progression to full-blown AD. It's clear that there is an urgent need for more and better drugs to treat AD, but there are few in the pipeline. To get insight into the nature of the problem researchers at the Cleveland Clinic Lou Ruvo Center for Brain Health, using the Clinicaltrials.gov website, conducted the first-ever analysis of clinical trials for Alzheimer's disease. Looking at a decade's worth of trials (2002-2012) they discovered that not only are there few AD drugs in development, but the failure rate for AD drug development is 99.6% and the number of drugs being studied has been declining since 2009. Only one new drug, memantine, has been approved since 2004.
Why is there a dearth of AD drug development? One of the Cleveland Clinic researchers, Jeffrey Cummings, explains in an interview with the Biome online magazine, "There are many contributing factors to the low number of trials. The investment of the U.S. National Institutes of Health (NIH) in Alzheimer's disease is relatively modest - $650 million compared to $6 billion in cancer, for example - so the rate of target identification is low. Similarly, many drugs are first discovered in academic laboratories and then out-licensed to biotechnology and pharmaceutical companies; the low rate of investment in discovery leads to few candidates to assess. At the biotechnology and pharmaceutical level, the high failure rate, long trials required, and high cost discourages investment in this area. Venture capital is less available in this area because of the high failure rates. Many biopharma groups have exited central nervous system drug development."
One way to help speed up AD drug development is, instead of starting from scratch, to conduct more repositioning studies, which take an already approved drug and aim to get it approved to treat a different condition. For example, researchers at the Lou Ruvo Center for Brain Health are leading a Phase IIa clinical trial to determine if bexatorene, a drug now approved to treat skin cancer, can remove a protein build-up in the brains of Alzheimer's patients. Increased government funding would, of course, also help encourage drug development.
The Cleveland Clinic researchers conclude their paper with the statement, "An urgent need exists to increase the number of agents entering the pipeline and progressing successfully toward new therapy for patients with AD." Although the researchers do not lay out a clear solution to the AD drug development problem, by clearly documenting the severity of the situation they may provoke action among those who can make a difference.
You May Also Like
Loading Comments...