In a
new study published in Nature, scientists describe a new protein family that are an integral part of the cell walls of bacteria. Study leaders David Rudner and Thomas Bernhardt suggest this group of enzymes might be a new avenue in the fight against harmful bacteria, an area of increasing importance as antibiotic resistance becomes more common.
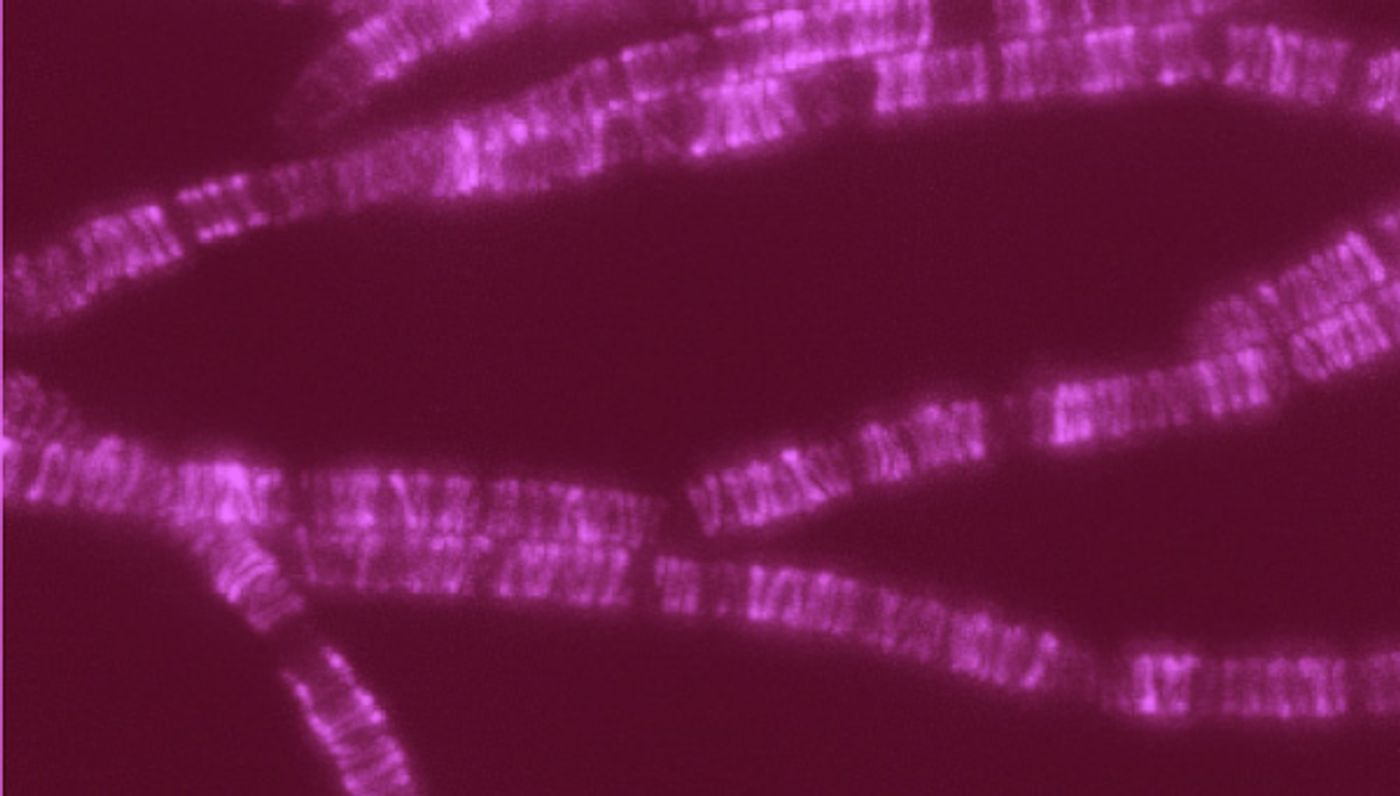
Lines in this colorized image of Bacillus subtilis reflect the movement of a newly identified polyermase complex (including the SEDS protein) as it synthesizes hoops of bacterial cell wall material. /Credit: Rudner lab
"We know these proteins are a great target because they are enzymes we can inhibit from the outside of the cell," explained Rudner, a Professor of Microbiology and Immunobiology at Harvard Medical School (HMS) and senior author of the work.
"Now we have a better handle on what these proteins do and how a potential drug might affect their activity," said Bernhardt, a co-author of the paper and HMS Professor of Microbiology and Immunobiology.
Cell walls are made of linked chains of sugars and are integral to a cells’ survival. A cell wall can keep out toxins and harmful invaders as well as maintain the shape of form of the cell.
In 1957, researchers discovered that penicillin worked by stopping the proteins that create bacterial cell walls. In the 1970s and 1980s, it was learned how those proteins that penicillin blocks work to build the cell wall. For a time, those proteins were assumed to be the primary drivers of cell wall synthesis in bacteria.
In 2003, investigators discovered that a bacterium, Bacillus subtilis, shown growing in the video above, could create a cell wall without those proteins that penicillin stops. That was a pretty big clue that another, unknown enzyme, sometimes referred to as the “moonlighting enzyme,” was at work on the cell wall.
Tsuyoshi Uehara, another co-author and a former HMS research fellow in the Bernhardt lab, had an idea for what the missing enzyme could be - the SEDS (shape, elongation, division and sporulation) family of proteins. The family is poorly characterized, yet known to be critical during cell growth, division and sporulation.
The research team removed all proteins that synthesize the cell wall and are known to bind penicillin; they observed SEDS proteins behaving normally. This indicated to the researchers that they were on the right track.
Further experimentation confirmed their hypothesis that the SEDS proteins are a cell wall synthesizing family. They were also able to demonstrate that the SEDS family builds hoop-like structures around the cell, and the penicillin-binding proteins fill in smaller strands to complete the cell wall.
![The SEDS proteins bear similarity to known glycosyltransferases [cell wall building enzymes] Schematic diagrams of the SEDS protein RodA and known glycosyltransferases from Gram-negative bacteria with their undecaprenyl pyrophosphate (undPP)-linked substrates and products. Credit: Nature Meeske at al](https://d3bkbkx82g74b8.cloudfront.net/eyJidWNrZXQiOiJsYWJyb290cy1hc3NldHMiLCJrZXkiOiJfcHVibGljXC9fZmlsZXNcL3N5c3RlbVwvY2tcL3RyZW5kaW5nXC9jZWxsd2FsbF80NGM2ODQ1MmUxMGE5YzM3MGRlMjcxMzliZmY4MjQ0Yi5qcGciLCJlZGl0cyI6eyJyZXNpemUiOnsid2lkdGgiOjE0MDAsImZpdCI6ImNvdmVyIn19fQ==)
The SEDS proteins bear similarity to known glycosyltransferases [cell wall building enzymes] Schematic diagrams of the SEDS protein RodA and known glycosyltransferases from Gram-negative bacteria with their undecaprenyl pyrophosphate (undPP)-linked substrates and products. Credit: Nature Meeske at al
"For a long time in the field, it was thought that one set of enzymes worked in one set of complexes to build a wall. Now we have two sets of enzymes appearing to work in different systems," Bernhardt explained. "Somehow they have to coordinate to build this netlike structure that maintains integrity and expands as the cells grow and divide."
SEDS proteins are also more common than the proteins that bind penicillin, and that might mean an antibiotic that targeted SEDS proteins could be more effective against a range of bacteria.
Sources:
AAAS/Eurekalert! via
Harvard Medical School,
Nature

![The SEDS proteins bear similarity to known glycosyltransferases [cell wall building enzymes] Schematic diagrams of the SEDS protein RodA and known glycosyltransferases from Gram-negative bacteria with their undecaprenyl pyrophosphate (undPP)-linked substrates and products. Credit: Nature Meeske at al](https://d3bkbkx82g74b8.cloudfront.net/eyJidWNrZXQiOiJsYWJyb290cy1hc3NldHMiLCJrZXkiOiJfcHVibGljXC9fZmlsZXNcL3N5c3RlbVwvY2tcL3RyZW5kaW5nXC9jZWxsd2FsbF80NGM2ODQ1MmUxMGE5YzM3MGRlMjcxMzliZmY4MjQ0Yi5qcGciLCJlZGl0cyI6eyJyZXNpemUiOnsid2lkdGgiOjE0MDAsImZpdCI6ImNvdmVyIn19fQ==)