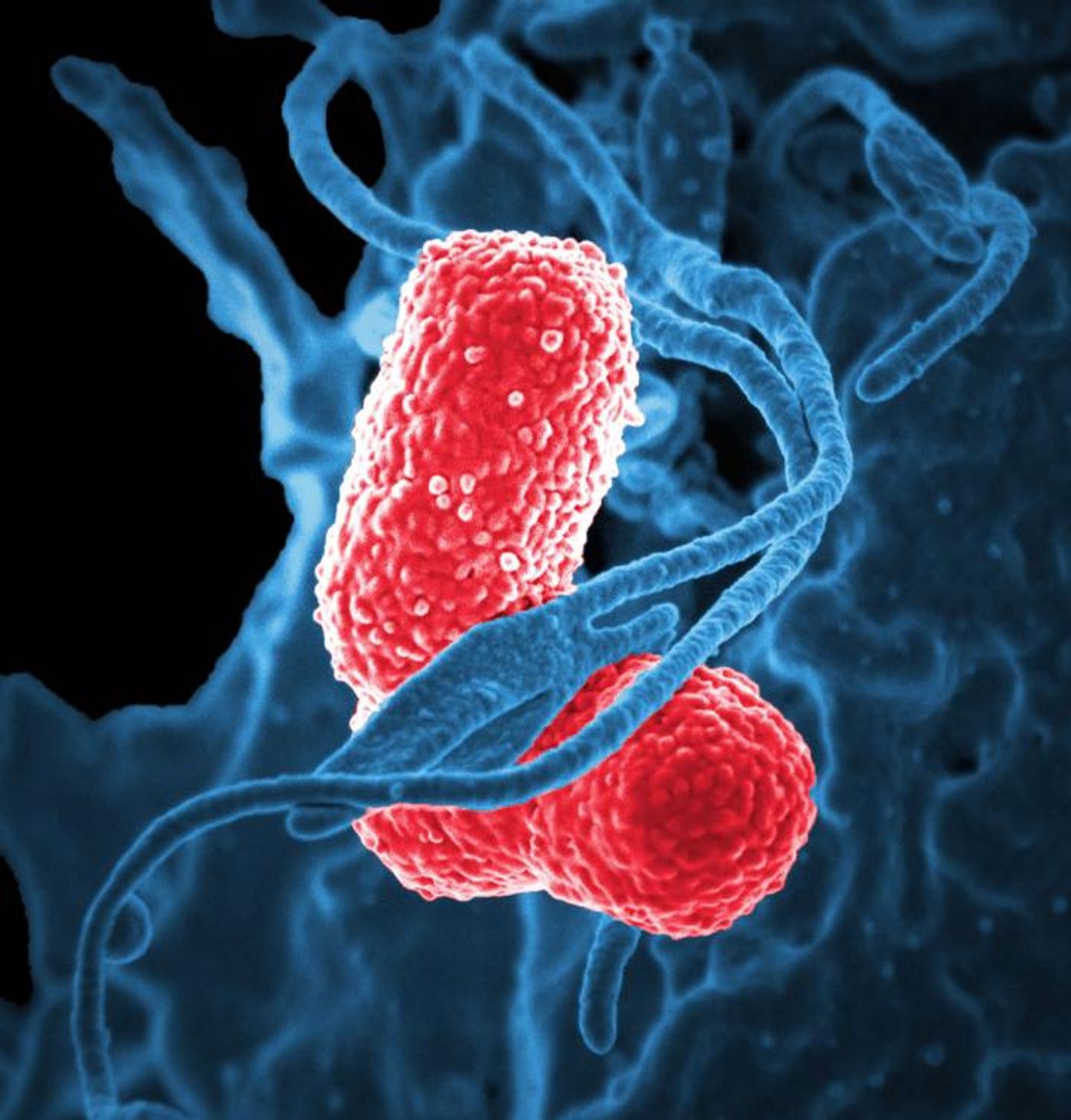
Klebsiella pneumoniae, AKA the “nightmare bacterium,” causes multiple infections including pneumonia, urinary tract infections, wound infections, and bloodstream infections, and the bacteria is frighteningly resistant to many drugs, including carbapenem. A new study aims to better understand superbugs like
K. pneumoniae, so scientists can learn to kill these bacteria better and faster.
Humans need iron to produce DNA, hemoglobin, and myoglobin, all necessary for life, respiration, and movement. However, bacteria like
K. pneumoniae also need iron to grow and multiply. When humans and
K. pneumoniae suddenly occupy the same space, the “arms race” for iron becomes very serious.
K. pneumoniae produce siderophores to do their dirty work for them. In fact, iron-binding siderophores from
K. pneumoniae are greatly superior to any human proteins that bind iron. In the new study, from the University of Michigan, researchers created bacteria that produced siderophores without actually reproducing themselves by preventing re-entrance of siderophores into bacterial cells after returning from their mission to obtain iron. By doing this in mice models of
K. pneumoniae, the researchers could finally see what the bacterial siderophores were doing and how their activity made
K. pneumoniae infections so damaging and resistant.
“It turns out that the mechanism [
K. pneumoniae] uses [to obtain iron] also causes cellular stress during infections," said Michael Bachman, MD, PhD. "That response triggers an immune response that tells our bodies to fight the infection, but it also activates a mechanism that allows bacteria to escape and travel to the rest of the body."
The immune response is triggered by siderophores, causing the release of inflammatory cytokines and other lymphocytes, but something was still inhibiting the host immune system from completely neutralizing and clearing the
K. pneumoniae infection. The protein to blame? Hif-1alpha.
In normal situations where the body experiences low blood oxygen or low blood iron, Hif-1alpha is called upon to restore supplies to a healthy level. However, when low blood iron caused by
K. pneumoniae siderophores activates Hif-1alpha, the bacterial infection only gets worse. While the current study helped bring this connection to light, researchers are still unsure why it happens.
When they eliminated Hif-1alpha in the lung cells of the mice, the infection didn’t spread like it did when Hif-1alpha was present. It could be that eliminating Hif-1alpha is the key to reducing cellular stress, correcting damage done to tissues from infection, and controlling the infection to a single location.
Other potential solutions being considered by the research team include:
- Preventing K. pneumoniae from sending out siderophores,
- Using siderophores to bring antibiotics back into bacterial cells,
- Creating a vaccine that targets siderophores, stimulates the immune system to target siderophores,
- And transposon sequencing to determine which bacterial genes from K. pneumoniae and other superbugs are required for infection.
As the third most common cause of infection for hospitalized patients,
K. pneumoniae is an important bacterial species to take down, and researchers are hopeful that the findings from their latest study will soon lead them to the best solution. The study was recently published in the journal
mBio.
Sources:
University of Michigan Health System,
Iron Disorders Institute