Novel Treatment Found to Overcome Therapy-Resistant Leukemia
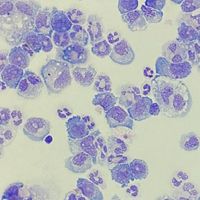
Acute myeloid leukemia (AML) is a rare and aggressive hematologic malignancy. AML progresses rapidly and is indicated by an excess of immature white blood cells. It is caused by high mutational burden over the span of a person’s life. One signature mutated gene includes the tumor suppressor gene TP53. Normally, TP53 helps make protein to stop oncogenesis or the formation of tumors. However, mutated TP53 loses that function and commonly results in AML. Unfortunately, those that have a TP53 mutation have an extremely aggressive tumor that is resistant to conventional chemotherapy drugs and results in poor prognosis. Other standard treatments include stem-cells transplants, and sometimes targeted drugs such as intracellular pathway inhibitors. Although many treatments are routine and help the patient reduce symptoms, there is no cure. Extensive research is currently being done by researchers and physicians to identify new approaches for AML treatment.
One novel therapy used in other hematologic malignancies includes chimeric antigen receptor (CAR)-T cell therapy. This therapy takes immune T cells (responsible for lysing or kill infections) from the patient or a donor and engineers them to target the tumor. Normally, these T cells would not recognize tumor growth, therefore, the engineered CAR-T cells are programmed to elicit an immune response and recognize surface markers on the tumor to lyse it. This therapy has been successful in other leukemias such as B-cell acute leukemia, and researchers are working to overcome treatment resistant AML using the same approach.
A recent article in EMBO Molecular Medicine, by Drs. Markus Manz, Stephen Boettcher and others, demonstrate that TP53-mutated AML is resistant to CAR-T cell therapy as a single agent, but can be overcome through combination therapy. Manz and Boettcher are principal investigators from the University of Zurich and the Department of Medical Oncology and Hematology at the University Hospital Zurich (USZ) and focus on mechanisms surrounding hematological diseases. The Zurich team first reported why TP53-mutated AML is resistant to CAR-T cell therapy. Using various models, it was noted that the engineered T cells quickly become ‘exhausted’ or inactive due to overstimulation or surrounding stimuli. The team further studied the underlying mechanism in this disease by concluding that TP53-deficient cells caused resistance through several metabolic pathways. Moreover, these pathways including the mevalonate and Wnt pathways were identified to improve therapeutic efficacy.
To overcome CAR-T cell resistance the team discovered that the mevalonate pathway was upregulated in the TP53-mutated AML cells. More work revealed that by blocking this pathway sensitizes AML to CAR-T cell therapy. Additionally, intracellular pathways within the T cells were also analyzed through transcriptomic profiling through gene expression. Researchers found that transcription factors involved in the Wnt signaling pathway were downregulated. The Wnt signaling pathway is essential for T cell development and function. Therefore, the team stimulated this pathway and found improve CAR-T cell function against AML. Overall, this work indicates that combination therapy is necessary to overcome therapy resistance in AML. It also identifies two therapeutic treatments to sensitize AML to treatment and improve CAR-T cell therapy. Consequently, the proposed therapeutic combinations have the potential to improve patient quality of life and significantly prolong survival.
Article, EMBO Molecular Medicine, Markus Manz, Stephen Boettcher, University of Zurich, USZ