Antibodies From Different People Tend to GRAB the Same Targets
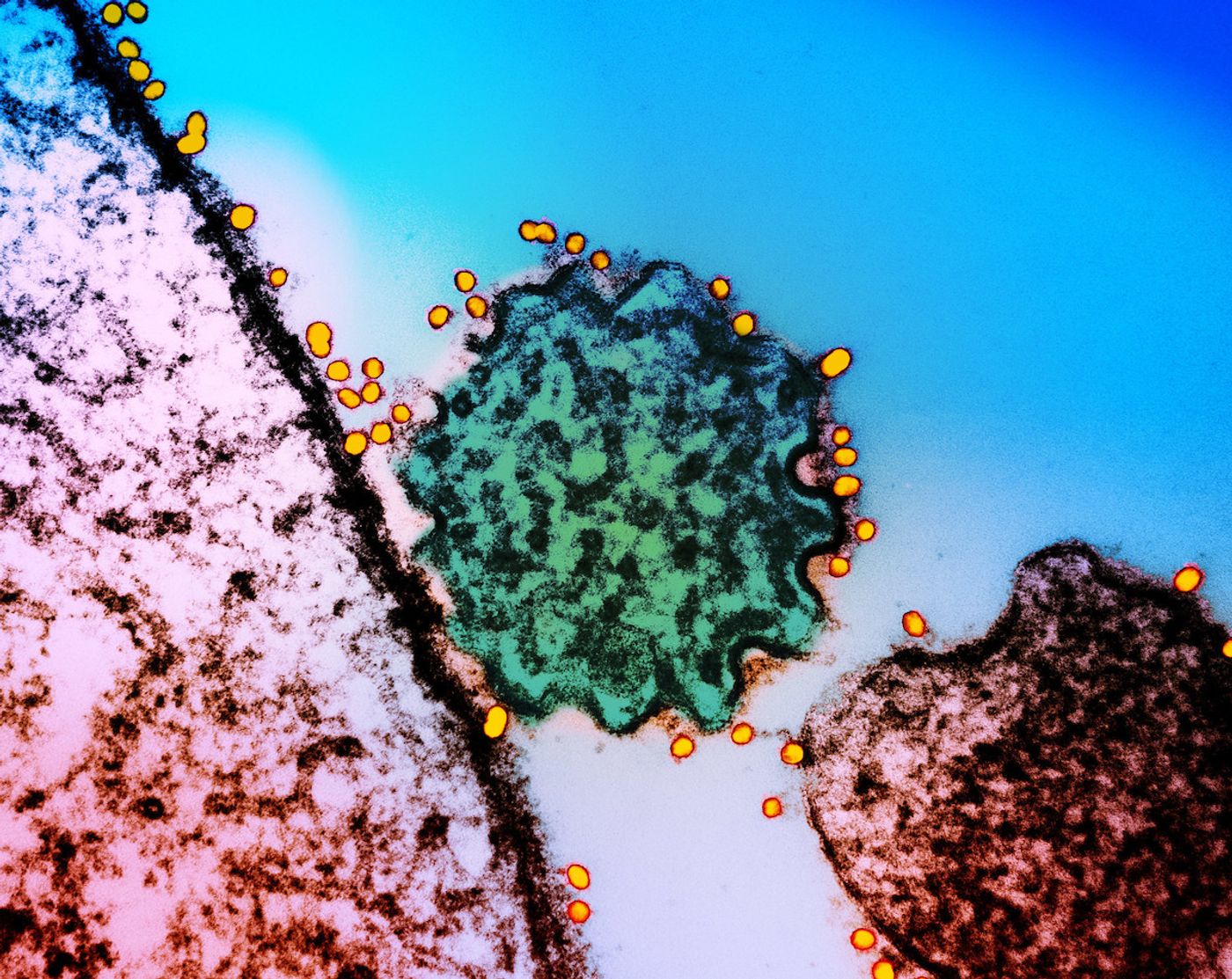
When the human body encounters a pathogen, it can generate a huge array of antibodies that aim to neutralize that pathogen. New research has suggested, however, that in the case of viruses the immune system tends to generate antibodies that target the same parts of the virus over and over. These so-called public epitopes, antibodies that are frequently found in many different people, may indicate that the production of antibodies is not a totally random process, and that viruses can easily evade these antibodies with mutations in individual amino acids. This work, which could have significant implications for public health and how we understand the immune system, have been reported in Science.
This study could explain reinfection patterns that have occurred during the COVID-19 pandemic, noted corresponding study author Stephen J. Elledge, PhD, the Gregor Mendel Professor of Genetics at Brigham and Women's Hospital and Harvard Medical School (HMS). “Our findings could help inform immune predictions and may change the way people think about immune strategies.”
It has previously been suggested that the human immune system does not target viral proteins randomly. The same antibody responses have been identified in different individuals; their antibodies are built to latch on to the same part of a viral protein or epitope, creating a kind of 'public epitope.' This study has shown that this could be a common phenomenon, and what the mechanisms behind it may be.
A tool created by the Elledge lab in 2015 called VirScan can identify thousands of places where antibodies bind to viruses, which uses one drop of blood to illustrate part of a person's immunological history. In this work, 569 blood samples from people in the US, Peru, and France were analyzed with VirScan. This revealed that the antibody response frequently targets the same viral regions. The researchers focused on 376 viral epitopes that were commonly targeted.
Antibodies seem to recognize public epitopes with bindings motifs that are encoded at birth; these germline-encoded amino acid binding (GRAB) motifs are experts at identifying one amino acid in particular. Antibodies geneeated by humans do not appear to select targets randomly, and instead tend to choose places where the GRAB motifs are good at binding. Thus, the same viral epitopes are repeatedly selected.
Viruses, meanwhile, only need to generate a few mutations to evade these common antibodies, so they can reinfect people and entire populations that were once immune.
Nonhuman species create antibodies that latch on to viral epitopes that are different from the ones that human immune systems select, noted the study authors. There are also some people who generate antibodies that do not match the public epitopes, and these individuals could have superior protection against reinfection compared to others. This information may help researchers formulate better treatments or vaccines for COVID-19.
“The more unique antibodies may be a lot harder to evade, which is important to consider as we think about the design of better therapies and vaccines,” said Elledge.
Sources: Brigham and Women's Hospital, Science