Stem Cell-Derived Neurons Reaching Maturity Thanks to Dynamic Nanofibers
Image Credit: Northwestern University
Imagine trying to learn about human physiology but only studying infants. For years, that’s what cellular-molecular neuroscientists have attempted with current stem cell culturing techniques. Growing induced pluripotent stem cells (iPSCs) into mature neurons has proved challenging, leaving researchers with immature neurons to model adult disease states.
Scientists at Northwestern University released findings on a new culturing technology that features an environment embedded with synthetic nanofibers and therapeutic peptides. Plated on this surface, cells from individuals with amyotrophic lateral sclerosis (ALS) were successfully differentiated into motor neurons and grew to the greatest level of maturity yet achieved.
The culture’s network of nanofibers is composed of many long ambiphilic molecules. While the hydrophobic ends point inward, the opposite end of the molecule presents the extracellular environment with various therapeutic signaling peptides, encouraging neuronal health.
The “hinge” to the whole dynamic is the molecule’s highly flexible center region, as it allows the peptide tips to rapidly flutter in the extracellular space..
This synthetic and therapeutic environment was developed in the laboratory of Samuel I. Stupp. His group optimized the supramolecular motion to mimic biological movement. More motion means more chances for the helpful peptides offered by the nanostructure to properly engage with neuron receptors. When injected at the site of spinal cord injury, flexible hinges encouraged spinal cord regeneration.
Now modified into a culturing surface, the internal motion of these nanostructures enhances the delivery of therapeutic cargo to growing neurons. In this new study, Stupp explained how they “confirmed that neurons coated with our nanofibers achieve more maturity than other methods, and mature neurons are better able to establish the synaptic connections that are fundamental to neuronal function” via EurekAlert!. Because the highly mature neurons displayed more in vivo neurons properties, this new technology has applications for both in vitro and patient therapy.
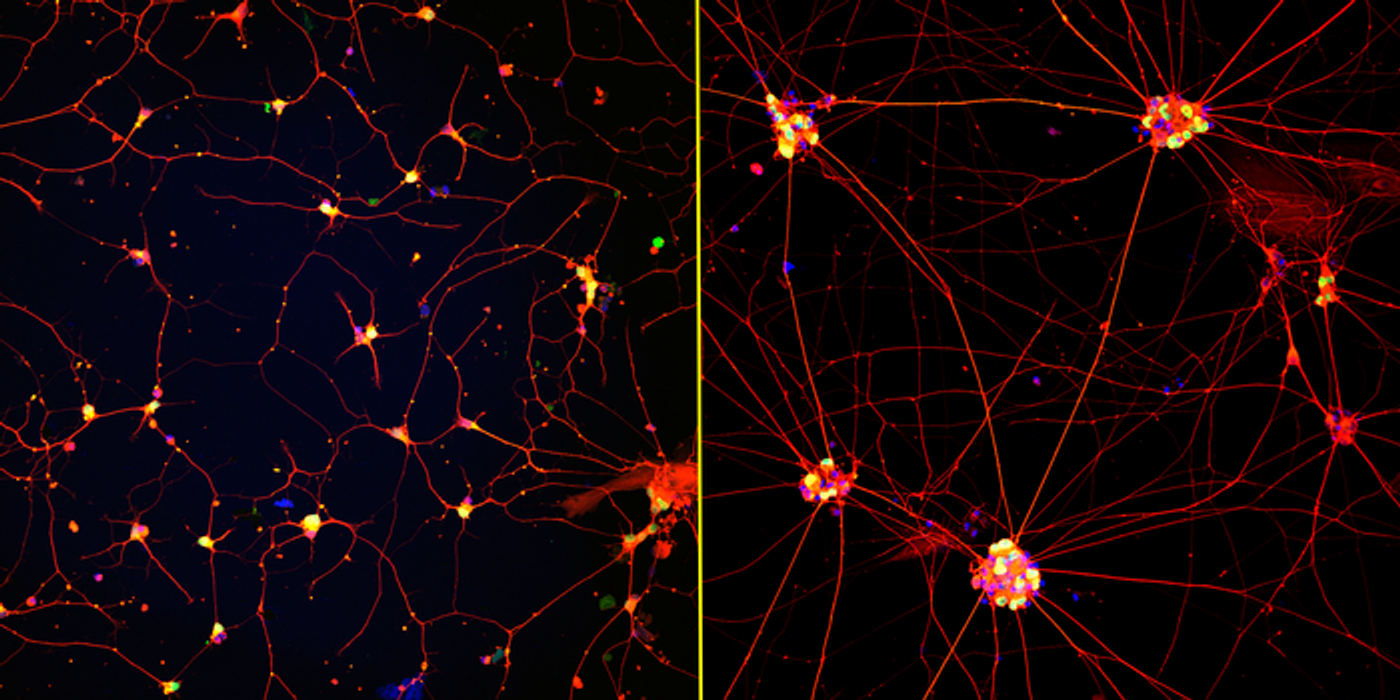
The in vitro dish model is cost-effective and applicable to later-onset diseases. The nanofiber-plated neurons spread across their environment’s surface (image left panel) instead of crowding together (image right panel). While the clumps were difficult to maintain, the new spreading has improved survival rates. Both scientists and cells are happier with how the mature neurons feature enhanced synaptic signaling, increased electrical activity, and greater dendritic arborization.
Advanced disease states are now being modeled. “For the first time, we have been able to see adult-onset neurological protein aggregation in the stem cell-derived ALS patient motor neurons,” said study author Evangelos Kiskinis via EurekAlert. While it’s still unclear how this aggregation is associated with the disease, Kiskinis is excited to investigate further using this newly developed technology.
Sources: Northwestern Now, Eureka Alert!, Science, Cell Stem Cell, Cell