Previously Unknown Membrane-Bound Organelles Key to C. difficile's Iron Defense
Clostridioides difficile (C. diff), a notorious spore-forming anaerobe, poses a significant global public health threat, claiming over 29,000 lives annually. Particularly menacing for those over 65, the mortality rate within 30 days of diagnosis stands at a staggering 1 in 9. However, a groundbreaking study led by researchers at Vanderbilt University, including Eric Skaar, PhD, MPH, the director of the Vanderbilt Institute for Infection, Immunology, and Inflammation, has uncovered a hidden aspect of C. diff's biology that could revolutionize our approach to combating this formidable pathogen.
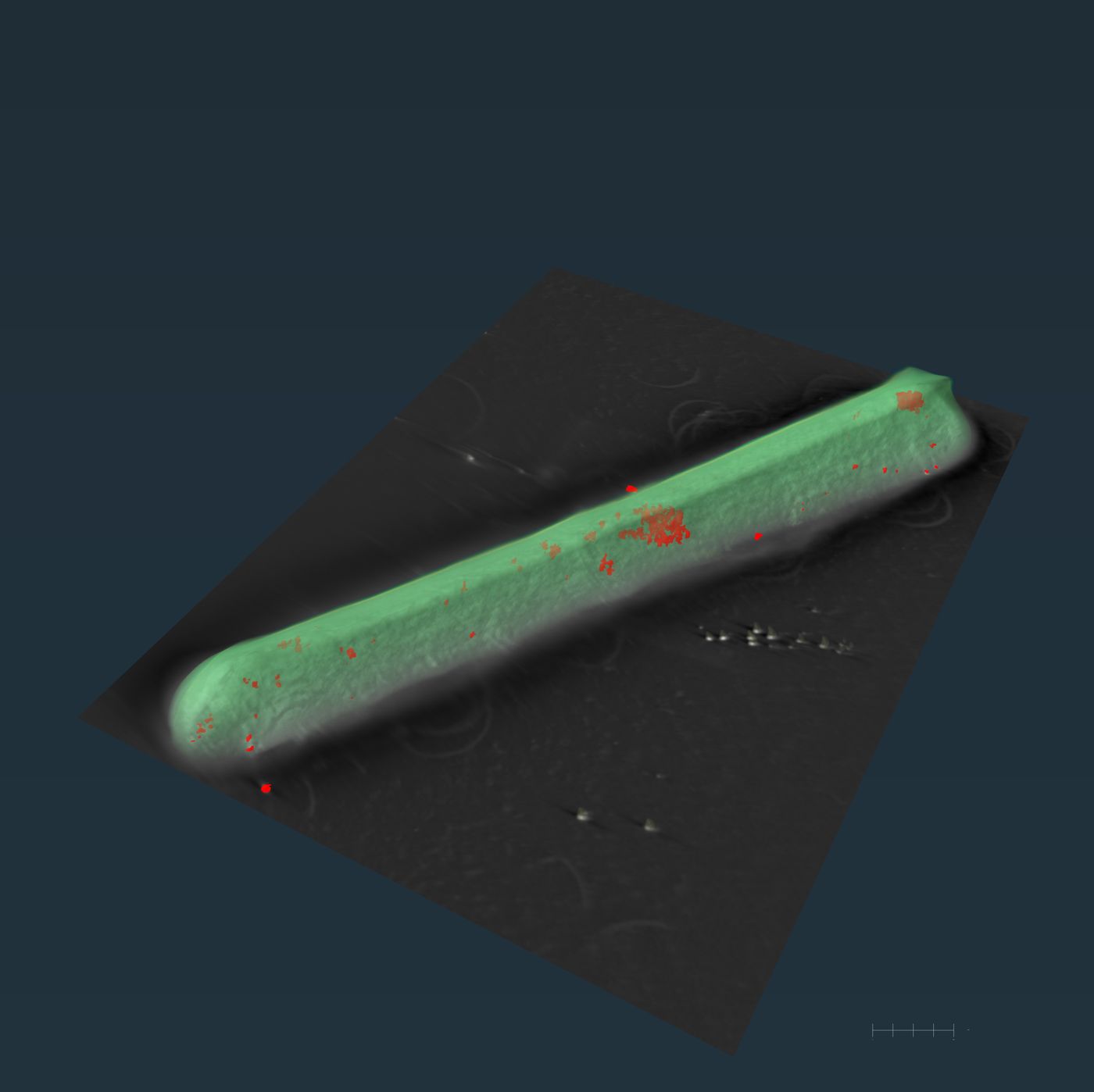
A C. diff bacterium (green) with iron particles in red, shown in a reconstructed electron tomogram from STEM-EDS. James McBride/Vanderbilt University
Trace metals, including zinc, manganese, and iron, are crucial for the function of nearly one-third of proteins across the microbe and animal kingdom. One defensive strategy against microbes is nutritional immunity, which can feature intoxication with metals or sequestering metals away from invading pathogens. Before this study, the specific mechanisms through which C. diff obtains iron or avoids iron intoxication were unknown.
Co-first authors Hualiang Pi, PhD, and Rong Sun, PhD and colleagues used STEM-ED technology to shine electron beams onto the pathogens and read the x-ray spectrum to determine the elements present. They discovered the accumulation of iron 'dots' within the bacterium.
They uncovered the previously unknown existence of a membrane-bound organelle within C. diff. Skaar underscores the importance of this discovery, noting that it "flips the field of microbiology on its head" by revealing the compartmentalization of biochemical processes in bacteria similar to eukaryotic cells.
C. diff employs a unique defense mechanism, producing iron phosphate-containing ferrosome organelles. These ferrosomes act as a shield, preventing iron overdose in environments contaminated with an excess of this essential nutrient.
The biological system surrounding these ferrosomes activates during infections, allowing C. diff to sidestep nutritional immunity. The researchers identified two essential genes, fezA and fezB, crucial for C. diff's pathogenicity in animal models. Skaar highlights the importance of this discovery, "Anytime we find new factors involved in host-pathogen interactions and show that they're important for infection, that opens entirely new opportunities to make classes of antibacterial drugs that have not existed before."
This research sheds light on the intricate strategies C. diff employs to evade nutritional immunity and presents a promising avenue for developing targeted antimicrobial therapies. With rising global antimicrobial resistance, identifying ferrosome formation as a potential target is a significant step forward.
The revelation of ferrosome organelles provides scientists with a fresh perspective to explore other subcellular compartments in bacteria and their role in physiological processes. Future drug research may discover ways to target these ferrosome organelles, offering a potential means to starve pathogens like C. diff of iron.
Sources: Nature Reviews Microbiology, Nature, EurekAlert!