Catalyst
Catalyst: is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. Catalysts may be classified as either homogeneous or heterogeneous. A homogeneous catalyst is one whose molecules are dispersed in the same phase (usually gaseous or liquid) as the reactant's molecules. A heterogeneous catalyst is one whose molecules are not in the same phase as the reactant's, which are typically gases or liquids that are adsorbed onto the surface of the solid catalyst. Enzymes and other biocatalysts are often considered as a third category.
-
JAN 22, 2018Genetics & GenomicsSynthetic biology is a growing field of research that is aiming to create a new kind of organism.Written By: Carmen LeitchJAN 18, 2018Chemistry & PhysicsAccording to a recently published study on the American Chemical Society journal ACS Catalysis, a team of engineering re ...Written By: Daniel DuanJAN 15, 2018CancerWith the help of a glow-producing natural enzyme, researchers detected the death of a single cancer cell quickly -- with ...Written By: Julia TraversJAN 10, 2018NeuroscienceIn the comic books and cartoons of the 1950s and 1960s, the future was where it was at. Flying cars, robot household hel ...Written By: Brenda Kelley KimDEC 20, 2017Chemistry & PhysicsThe sun is often seen as an under-utilized source of energy. Solar energy is abundant, reliable and pollution free, but ...Written By: Daniel DuanNOV 21, 2017TechnologyFoldit is a computer game that uses human ingenuity and "problem-solving intuition" to explore the behaviors of proteins ...Written By: Julia TraversNOV 15, 2017TechnologySophia was interviewed at Web Summit, a tech conference in Portugal, in Nov. 2017.Written By: Julia TraversSEP 18, 2017Space & AstronomyAstronomers from both McGill University in Canada and the University of Exeter in the U.K. used the Hubble Space Telesco ...Written By: Anthony BouchardSEP 10, 2017VideosAs of early September, a new cancer treatment has been approved to treat acute lymphoblastic leukemia. The treatment is ...Written By: Kathryn DeMuth SullivanSEP 09, 2017Plants & AnimalsMonarch butterflies are among some of the world’s most precious insects, not just because they’re beautiful ...Written By: Anthony BouchardSEP 05, 2017Space & AstronomyAsk almost anyone you know what supernovae are, and you’ll probably hear how they’re the result of a star co ...Written By: Anthony BouchardAUG 27, 2017Chemistry & PhysicsThe reaction mechanism that is responsible for NOx catalytic reduction in diesel engine emission-control process. Credit ...Written By: Daniel DuanAUG 07, 2017Plants & AnimalsFrogs are among some of the most diverse vertebrates on the planet, but their origins have long been cloudy. On the othe ...Written By: Anthony BouchardAUG 05, 2017Chemistry & PhysicsA joint team of chemists from UC Berkeley and Lawrence Berkeley National Laboratory came up with a novel method for addi ...Written By: Daniel DuanJUL 09, 2017VideosAs summer heats up, you've probably noticed more fireflies in your backyard. These creatures are most visible at the dus ...JUN 03, 2017Plants & AnimalsClimate change can impact our environment in various ways, and as we’ve experienced first-hand with climate change ...Written By: Anthony BouchardMAY 20, 2017VideosIdeally, most of the world wants to transition to renewable energy to reduce our dependence on energy sources that aren' ...Written By: Anthony BouchardMAY 02, 2017VideosWhen it comes to finding alternatives for fossil fuels, it could be argued that the auto industry has been a little late ...Written By: Brenda Kelley KimAPR 14, 2017Chemistry & PhysicsInitial Demonstrations Yield Compounds with Promise for Treating COPD, Cystic Fibrosis and Other Lung Disorders Co-autho ...Written By: The Scripps Research Institute (TSRI)APR 14, 2017Earth & The EnvironmentAlgal biomass production is taking flight in the biotech world and recent results from the Tokyo Institute of Technology ...Written By: Kathryn DeMuth SullivanMAR 21, 2017VideosThere's nothing like a fresh tomato, picked right off the vine. The flavor is at its peak, and it's full of important nu ...Written By: Brenda Kelley KimMAR 14, 2017CancerWhen medical professionals are targeting a patient’s cancer, the goal is first to eliminate the cancer and if that ...Written By: Brenda Kelley KimMAR 08, 2017Chemistry & PhysicsLA JOLLA, CA – March 8, 2017 – Chemists at The Scripps Research Institute (TSRI) have devised a versatile mo ...Written By: The Scripps Research Institute (TSRI)FEB 02, 2017Chemistry & PhysicsLA JOLLA, CA – February 2, 2017 – Chemists at The Scripps Research Institute (TSRI) have invented a new tech ...Written By: The Scripps Research Institute (TSRI)
JAN 22, 2018
Genetics & Genomics
Synthetic biology is a growing field of research that is aiming to create a new kind of organism.
Written By:
Carmen Leitch
JAN 18, 2018
Chemistry & Physics
According to a recently published study on the American Chemical Society journal ACS Catalysis, a team of engineering re
...
Written By:
Daniel Duan
JAN 15, 2018
Cancer
With the help of a glow-producing natural enzyme, researchers detected the death of a single cancer cell quickly -- with
...
Written By:
Julia Travers
JAN 10, 2018
Neuroscience
In the comic books and cartoons of the 1950s and 1960s, the future was where it was at. Flying cars, robot household hel
...
Written By:
Brenda Kelley Kim
DEC 20, 2017
Chemistry & Physics
The sun is often seen as an under-utilized source of energy. Solar energy is abundant, reliable and pollution free, but
...
Written By:
Daniel Duan
NOV 21, 2017
Technology
Foldit is a computer game that uses human ingenuity and "problem-solving intuition" to explore the behaviors of proteins
...
Written By:
Julia Travers
NOV 15, 2017
Technology
Sophia was interviewed at Web Summit, a tech conference in Portugal, in Nov. 2017.
Written By:
Julia Travers
SEP 18, 2017
Space & Astronomy
Astronomers from both McGill University in Canada and the University of Exeter in the U.K. used the Hubble Space Telesco
...
Written By:
Anthony Bouchard
SEP 10, 2017
Videos
As of early September, a new cancer treatment has been approved to treat acute lymphoblastic leukemia. The treatment is
...
Written By:
Kathryn DeMuth Sullivan
SEP 09, 2017
Plants & Animals
Monarch butterflies are among some of the world’s most precious insects, not just because they’re beautiful
...
Written By:
Anthony Bouchard
SEP 05, 2017
Space & Astronomy
Ask almost anyone you know what supernovae are, and you’ll probably hear how they’re the result of a star co
...
Written By:
Anthony Bouchard
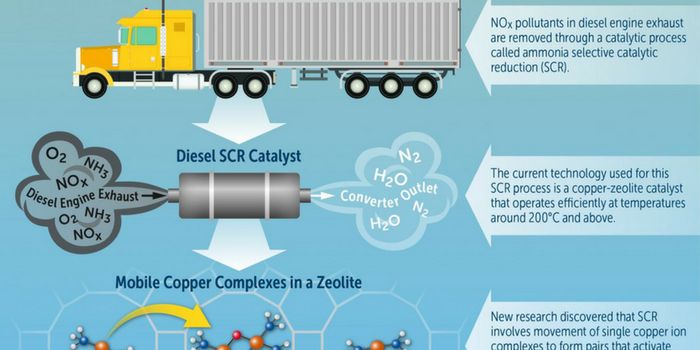
AUG 27, 2017
Chemistry & Physics
The reaction mechanism that is responsible for NOx catalytic reduction in diesel engine emission-control process. Credit
...
Written By:
Daniel Duan
AUG 07, 2017
Plants & Animals
Frogs are among some of the most diverse vertebrates on the planet, but their origins have long been cloudy. On the othe
...
Written By:
Anthony Bouchard
AUG 05, 2017
Chemistry & Physics
A joint team of chemists from UC Berkeley and Lawrence Berkeley National Laboratory came up with a novel method for addi
...
Written By:
Daniel Duan
JUL 09, 2017
Videos
As summer heats up, you've probably noticed more fireflies in your backyard. These creatures are most visible at the dus
...
JUN 03, 2017
Plants & Animals
Climate change can impact our environment in various ways, and as we’ve experienced first-hand with climate change
...
Written By:
Anthony Bouchard
MAY 20, 2017
Videos
Ideally, most of the world wants to transition to renewable energy to reduce our dependence on energy sources that aren'
...
Written By:
Anthony Bouchard
MAY 02, 2017
Videos
When it comes to finding alternatives for fossil fuels, it could be argued that the auto industry has been a little late
...
Written By:
Brenda Kelley Kim
APR 14, 2017
Chemistry & Physics
Initial Demonstrations Yield Compounds with Promise for Treating COPD, Cystic Fibrosis and Other Lung Disorders Co-autho
...
Written By:
The Scripps Research Institute (TSRI)
APR 14, 2017
Earth & The Environment
Algal biomass production is taking flight in the biotech world and recent results from the Tokyo Institute of Technology
...
Written By:
Kathryn DeMuth Sullivan
MAR 21, 2017
Videos
There's nothing like a fresh tomato, picked right off the vine. The flavor is at its peak, and it's full of important nu
...
Written By:
Brenda Kelley Kim
MAR 14, 2017
Cancer
When medical professionals are targeting a patient’s cancer, the goal is first to eliminate the cancer and if that
...
Written By:
Brenda Kelley Kim
MAR 08, 2017
Chemistry & Physics
LA JOLLA, CA – March 8, 2017 – Chemists at The Scripps Research Institute (TSRI) have devised a versatile mo
...
Written By:
The Scripps Research Institute (TSRI)
FEB 02, 2017
Chemistry & Physics
LA JOLLA, CA – February 2, 2017 – Chemists at The Scripps Research Institute (TSRI) have invented a new tech
...
Written By:
The Scripps Research Institute (TSRI)